Introduction:
The integration of immune checkpoint inhibitors (ICPIs) into cancer management has brought a paradigm shift in the outcome of cancer care. The incidence of hematological immune-related adverse events (Hem-irAEs) associated with ICIs is uncommon or exceedingly rare, particularly in comparison to chemotherapy (Johar, 2020). In this study, we provide an analysis of real-world experience with Hem-irAEs from the National Center of Cancer Care and Research (NCCCR) in Qatar.
Methods:
Patient electronic medical records (EMR) were retrospectively analyzed for all patients who received ICPIs between 2015 and 2020. Collected data included patient demographics, Hem-irAEs-related incidence, characterization, management, and outcomes of Hem-irAEs.
Results
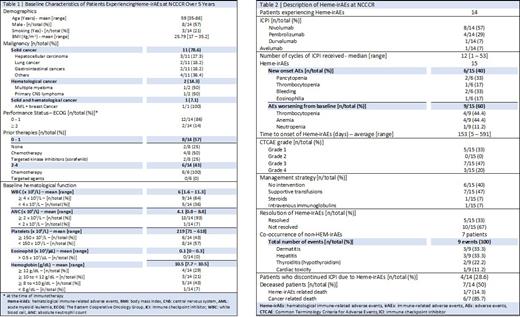
Over the five-year review period, a total of 165 irAEs were experienced among the 256 patients who received ICPIs in Qatar. The incidence of Hem-irAEs was 15 events (9.1% of all irAEs) among 14 patients (5.5% of all patients). Baseline characteristics of patients experiencing Hem-irAEs are described in Table 1. Majority of patients were diagnosed with solid malignancies (n=11; 78.6%) and were pre-treated with either chemotherapy or targeted therapies (n=12; 86%). Baseline hematological functions were also acceptable in most patients as shown in Table 1.
Nivolumab was the most used ICPI (n=8, 57%), followed by pembrolizumab (n=4, 29%), durvalumab (n=1, 7%) and avelumab (n=1, 7%). The most common Hem-irAE was thrombocytopenia (5/15); however, four of those events were worsening of baseline thrombocytopenia (Table 2). During this review period, two bleeding events have occurred; one was grade 1 gingival bleeding that spontaneously resolved, while the other event was grade 4 leading to severe anemia (hemoglobin 5.7 g/dL) requiring transfusion and ICPI discontinuation. Two grade 4 pancytopenia events were identified; one resolved with supportive transfusions and ICPI discontinuation, while the other resulted in fatal limbic encephalitis despite the use of intravenous immunoglobulins. There were no other fatal irAEs, but one more patient discontinued ICPI due to grade 3 anemia. Co-occurrence of non-HEM-irAEs were manifested in half of this population (n=7); majority of which were dermatitis (33.3%) and hepatitis (33.3%), followed by hypothyroidism (22.2%) and cardiac toxicity (11.2%).
Conclusion
Compared to the current literature describing Hem-irAEs, our center experience shows similar distribution of adverse events with thrombocytopenia and anemia. Thrombocytopenia was the most Hem-irAEs experienced followed by neutropenia and eosinophilia (1). On the other hand, our population is slightly different with the majority of cases described here being non-melanoma solid malignancies. Considering the rate of Hem-irAEs in the investigated population at NCCCR, there is a need for an integrated pathway for the diagnosis and management of these events.
Disclosures
No relevant conflicts of interest to declare.